Engineered M-MLV Reverse Transcriptase Basic Kit includes a rationally designed M-MLV RT that exhibits increased thermal stability and reduced RNase H activity (RNase H–) than the wild type M-MLV RT.
Please contact our exclusive distributor, Lab Supplies Scientific
Engineered M-MLV Reverse Transcriptase Basic Kit includes a rationally designed M-MLV RT that exhibits increased thermal stability and reduced RNase H activity (RNase H–) than the wild type M-MLV RT.
Source:
Gene coding the respective variant is heterologously expressed in E. coli.
Unit Definition:
One unit is defined as the amount of enzyme needed to catalyze the incorporation of 1 nmol dTTP into acid-insoluble material in 10 min under 37°C with poly(rA) and oligo(dT).
Reagents supplied:
| Cat number: RN012S kit | ||
| Cat No (color code) | Description | Tubes No. |
| RN001S | Engineered MMLV Reverse transcriptase, 200u/μl, 0.05ml
(200 units/μl in 20 mM Tris-HCl pH 7.4, 100 mM NaCl, 0.1 mM EDTA, 50 % glycerol, 0.01% IGEPAL, 1mM DTT) |
1 |
| BR055-1 | 5x Reverse Transcriptase buffer, 1ml
(250 mM Tris-HCl (pH 8.3), 375 mM KCl, 15 mM MgCl2) |
1 |
| BR056-0.5 | 100mM DTT, 0.5ml | 1 |
| BR028-0.15 | dNTPs mix 10mM each, 0.15ml | 1 |
| BR033-1 | Nuclease Free Water, 1 ml | 1 |
Reaction guidelines and tips:
Quantities per Reaction (20μL Final Reaction Volume):
| 1. Preparation of Mix A | 1 ng -2 μg of total RNA*
2 μL oligod(T)18 from 50 μΜ stock §, §§ 1 μl dNTPs mix from 10 mM each stock X μl of Nuclease Free Water to 10 μl |
| 2. Incubate Mix A at 65ο C for 5 min, then transfer for 1 min on ice (meanwhile prepare Mix B) | |
| 3. Preparation of Mix B | 2,5 μl of Nuclease Free Water
4 μl from 5x RT Buffer 2 μl DTT from 100 mM stock 0,5 μl of RNase inhibitor** 1 μL of Reverse Transcriptase enzyme from stock of 200 U/μl |
| 4. Following Step 2, add 10 μL of Mix B to the reaction | |
| 5. Incubate at 37ο C to 55ο C for 15-60 min *** | |
| 6. Heat inactivate the Reverse Transcriptase at 65ο C for 20 min | |
| 7. Cool down the cDNA to room temperature and use 0,1 – 2 μl in PCR reaction | |
* ≤ 0,1 ng may suffice for detecting certain RNA populations that are abundant, like e.g. Rubisco transcript in plants. Use 10 times less RNA quantity for RNAs enriched with coding transcripts e.g. passed through oligo(dT) beads
** The final concentration of the RNase Inhibitor is 1 U/μL. Since RT enzymes and reagents have been checked for the presence of RNases and DNases, this step may be omitted if the sample is of high RNA quality.
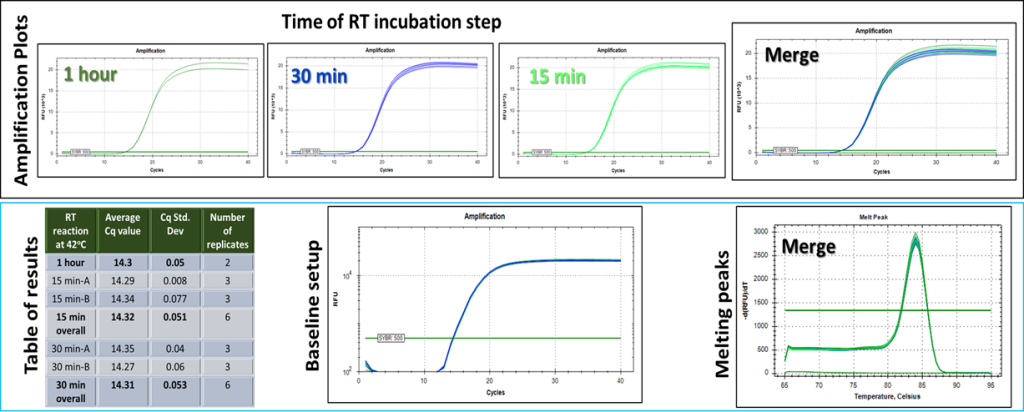
*** Routine temp for a standard reaction is 42ο C. For most RNA cases tested, complete cDNA production was observed within 10-15 minutes independently of the incubation temperature. Fell free to optimize depending on your target and provide us with your feedback to get special offers.
§ In case of random hexamers, add 2 μl from 50 μM stock per 20 μl RT reactions. Following “step2” incubate for 5 minutes at 25ο C or room temperature before incubating the reaction to 42ο C.
§§ In case of gene specific primer, add 2 μl from 2 μΜ primer stock. Incubate at 50ο C for cDNA production. Optimize your case depending on primer’s Tm. Incorporate 5-10 degrees below the Tm of your oligo. Do not incubate at temperatures higher than 55ο C.
Functional Quality Control:
Two step cDNA synthesis reaction in order to amplify an 831-bp region using 1ng Hela RNA as substrate. The resulting PCR product is visualized as a single band on a Midori-stained agarose gel.
Other Quality Controls
Tested extensively for the absence of DNases and RNases and E.coli DNA contamination.
Shipping
Shipped on blue ice or dry ice
Storage conditions
Store at -20οC ± 5οC
Shelf life:
24 months post production
Low copy gene*: 7.8 nTPM in HeLa cells (based on Human Atlas website)
High copy gene*: 5924.3 nTPM in HeLa cells (based on Human Atlas website)
*Expression levels’ annotation (low and high) has been performed according to the Expression Atlas website (EMBL-EBI)
(https://www.ebi.ac.uk/gxa/FAQ.html), nTPM : normalized Transcripts per Million
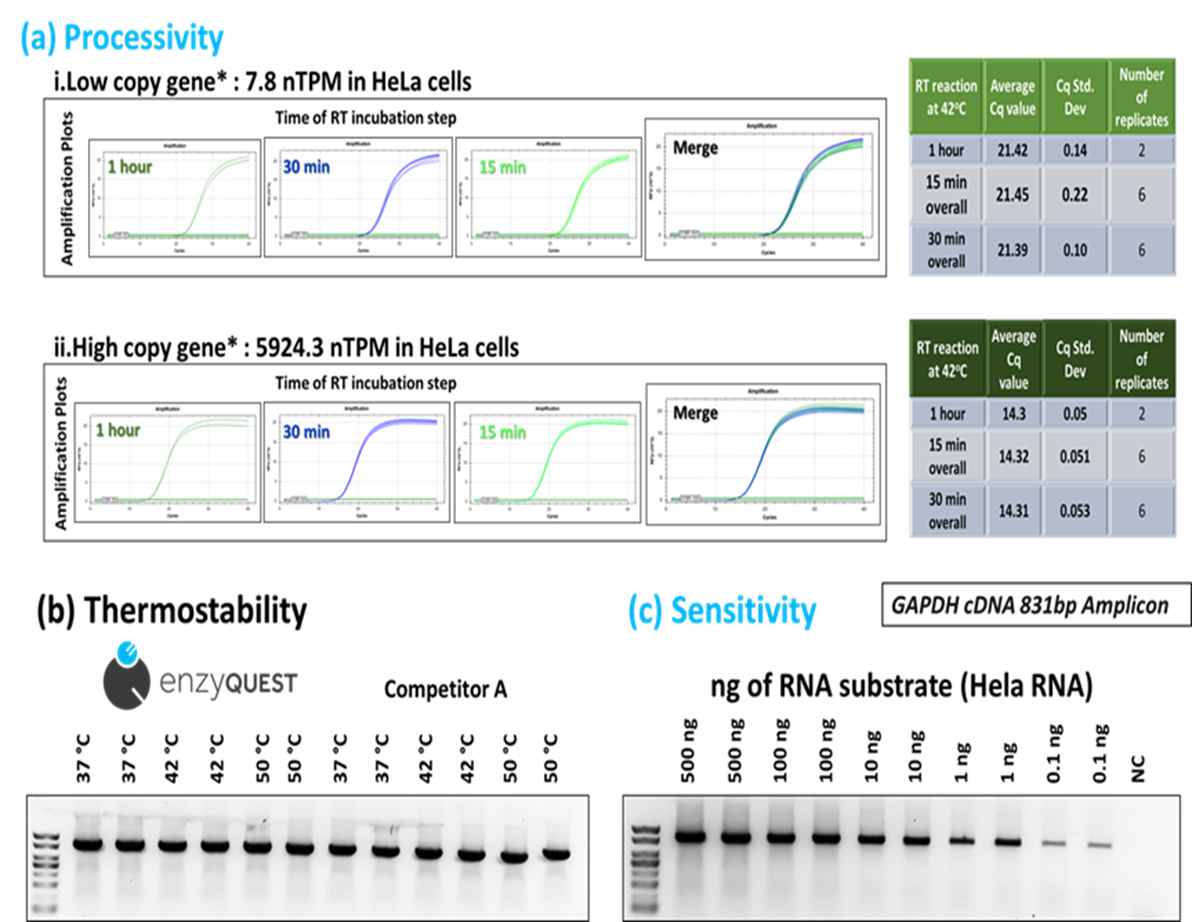
Processivity (a), thermostability (b) and sensitivity (c) down to 0.1 ng of total RNA per reaction. 100 ng of total RNA has been used for the thermostability tests. Sensitivity test RT reactions were incubated at 42 oC . In b and c, RT step was set at 15 minutes.
This engineered variant exhibits increased thermal stability (remains active up to 50°C) and reduced RNase H activity compared to wild-type M-MLV RT. This results in increased cDNA length and yield, and it can amplify regions up to 8 kb.
You can use three types of primers: (1) oligo(dT)18 (2 μl from 50 μM stock) for mRNA, (2) random hexamers (2 μl from 50 μM stock) for total RNA, or (3) gene-specific primers (2 μl from 2 μM stock) for targeted cDNA synthesis.
The RNase inhibitor is optional. The protocol recommends 0.5 μl (final concentration 1 U/μL), but since the RT enzyme and reagents have been checked for RNases and DNases, this step may be omitted if your RNA sample is of high quality.
The kit includes: (1) Engineered MMLV Reverse Transcriptase (200 U/μl), (2) 5x Reverse Transcriptase Buffer, (3) 100mM DTT, (4) dNTPs mix (10mM each), and (5) Nuclease Free Water. The kit contains enough reagents for 50 reactions.
After completing the reverse transcription reaction (20 μl total volume), use 0.1-2 μl of the cDNA product in your PCR reaction. The amount will depend on your target abundance and PCR optimization.